Drug discovery and development timeline
Construct the ideal formula with our Drug Discovery And Development Timeline. Ensure all components fit exactly.
Construct the ideal formula with our Drug Discovery And Development Timeline. Ensure all components fit exactly.
- Google Slides is a new FREE Presentation software from Google.
- All our content is 100% compatible with Google Slides.
- Just download our designs, and upload them to Google Slides and they will work automatically.
- Amaze your audience with SlideTeam and Google Slides.
-
Want Changes to This PPT Slide? Check out our Presentation Design Services
- WideScreen Aspect ratio is becoming a very popular format. When you download this product, the downloaded ZIP will contain this product in both standard and widescreen format.
-

- Some older products that we have may only be in standard format, but they can easily be converted to widescreen.
- To do this, please open the SlideTeam product in Powerpoint, and go to
- Design ( On the top bar) -> Page Setup -> and select "On-screen Show (16:9)” in the drop down for "Slides Sized for".
- The slide or theme will change to widescreen, and all graphics will adjust automatically. You can similarly convert our content to any other desired screen aspect ratio.
Compatible With Google Slides

Get This In WideScreen
You must be logged in to download this presentation.
PowerPoint presentation slides
Presenting this set of slides with name - Drug Discovery And Development Timeline. This is a four stage process. The stages in this process are Drug Discovery, Science, Medical.
People who downloaded this PowerPoint presentation also viewed the following :
Content of this Powerpoint Presentation
Description:
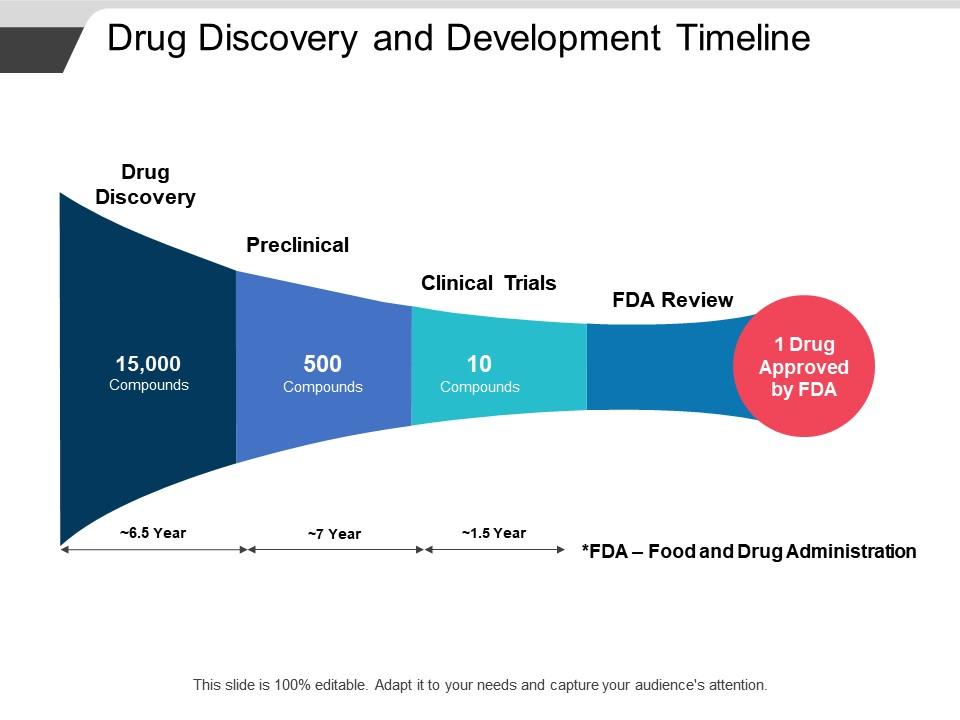
The image is a PowerPoint slide titled "Drug Discovery and Development Timeline," which illustrates the typical stages and duration of developing a new pharmaceutical drug. The timeline is segmented into four phases:
1. Drug Discovery:
This initial phase involves screening approximately 15,000 compounds over an estimated period of 6.5 years to identify potential candidates for development.
2. Preclinical:
Out of the initial pool, around 500 compounds are selected to undergo preclinical testing. This stage typically spans about 7 years and includes laboratory and animal testing to evaluate safety.
3. Clinical Trials:
From the preclinical phase, roughly 10 compounds move on to clinical trials, which last around 1.5 years. These trials are conducted with human participants to assess efficacy and safety.
4. FDA Review:
Finally, the process culminates with the FDA review, where on average, only 1 drug is approved. The footnote clarifies that "FDA" stands for Food and Drug Administration.
Use Cases:
Drug development timeline is relevant and can be used across several industries related to pharmaceuticals and healthcare:
1. Pharmaceuticals:
Use: Educating on drug development processes
Presenter: R&D Director
Audience: Research scientists, new employees
2. Biotechnology:
Use: Illustrating the timeline for biotech drug development
Presenter: Biotech Project Manager
Audience: Investors, stakeholders
4. Healthcare Consulting:
Use: Advising healthcare businesses on product lifecycles
Presenter: Healthcare Consultant
Audience: Healthcare executives, strategy teams
5. Medical Education:
Use: Teaching students about the drug approval process
Presenter: Academic Professor
Audience: Medical and pharmacy students
6. Healthcare Investment:
Use: Assessing the timeline for drug market entry
Presenter: Financial Analyst
Audience: Investors, financial advisors
7. Regulatory Affairs:
Use: Training on the regulatory aspects of drug development
Presenter: Regulatory Affairs Specialist
Audience: Regulatory affairs teams, compliance officers
8. Healthcare Marketing:
Use: Preparing marketing strategies based on drug development stages
Presenter: Marketing Manager
Audience: Marketing teams, brand strategists
Drug discovery and development timeline with all 5 slides:
Facilitate joyful events with our Drug Discovery And Development Timeline. Invite folks to indulge in fun filled activities.
No Reviews