Health Information Management Powerpoint Presentation Slides
Take advantage of our thoughtfully designed PowerPoint Presentation on Health Information Management, which offers a concise overview of digital biomarkers. This Digital Medicine PPT template provides a comprehensive introduction to digital biomarkers, including their definition, the underlying principles of behavioral analysis, and their significance in healthcare. Furthermore, the biomarker classification section presents the global market size, key market influencers, and market segmentation for digital biomarkers. Additionally, the Digital Health PowerPoint highlights the transformative impact of digital technologies in healthcare, exploring the benefits, implications, and application areas. It delves into the categorization of digital biomarkers and their user base. Our Biomedical Informatics template covers the latest sensing technologies and devices employed in digital biomarkers, such as sweat detection, insole sensors, disease prediction apps, and portable intelligent devices. Furthermore, the Wearable Sensors deck includes potential use cases, challenges, and solutions related to adopting digital biomarkers while comparing their characteristics to traditional biomarkers. Lastly, the Health Information Management PowerPoint encompasses a roadmap, a timeline for developing digital biomarker solutions, and a performance tracking dashboard. Access this invaluable resource now to stay ahead in the field of digital biomarkers.
You must be logged in to download this presentation.
PowerPoint presentation slides
Deliver this complete deck to your team members and other collaborators. Encompassed with stylized slides presenting various concepts, this Health Information Management Powerpoint Presentation Slides is the best tool you can utilize. Personalize its content and graphics to make it unique and thought-provoking. All the sixty nine slides are editable and modifiable, so feel free to adjust them to your business setting. The font, color, and other components also come in an editable format making this PPT design the best choice for your next presentation. So, download now.
People who downloaded this PowerPoint presentation also viewed the following :
Content of this Powerpoint Presentation
Slide 1: This slide introduces Health Information Management. State your company name and begin.
Slide 2: This is an Agenda slide. State your agendas here.
Slide 3: This slide shows Table of Content for the presentation.
Slide 4: This slide also shows Table of Content for the presentation.
Slide 5: This slide shows title for topics that are to be covered next in the template.
Slide 6: This slide describes the introduction to digital biomarkers that are transforming the healthcare system.
Slide 7: This slide depicts the future of digital biomarkers.
Slide 8: This slide presents the principles of behavioral analysis using digital biomarkers.
Slide 9: This slide describes how to separate direct digital biomarkers from indirect digital biomarkers.
Slide 10: This slide shows title for topics that are to be covered next in the template.
Slide 11: This slide outlines how digital biomarkers capture clinically meaningful and objective information cost-effectively.
Slide 12: This slide presents how digital biomarkers turn the evidence creation and validation process into a closed loop.
Slide 13: This slide describes how combining digital biomarkers allow for identifying phenotypic characteristics.
Slide 14: This slide shows title for topics that are to be covered next in the template.
Slide 15: This slide represents the global market size of the digital biomarkers from the year 2022 to 2028.
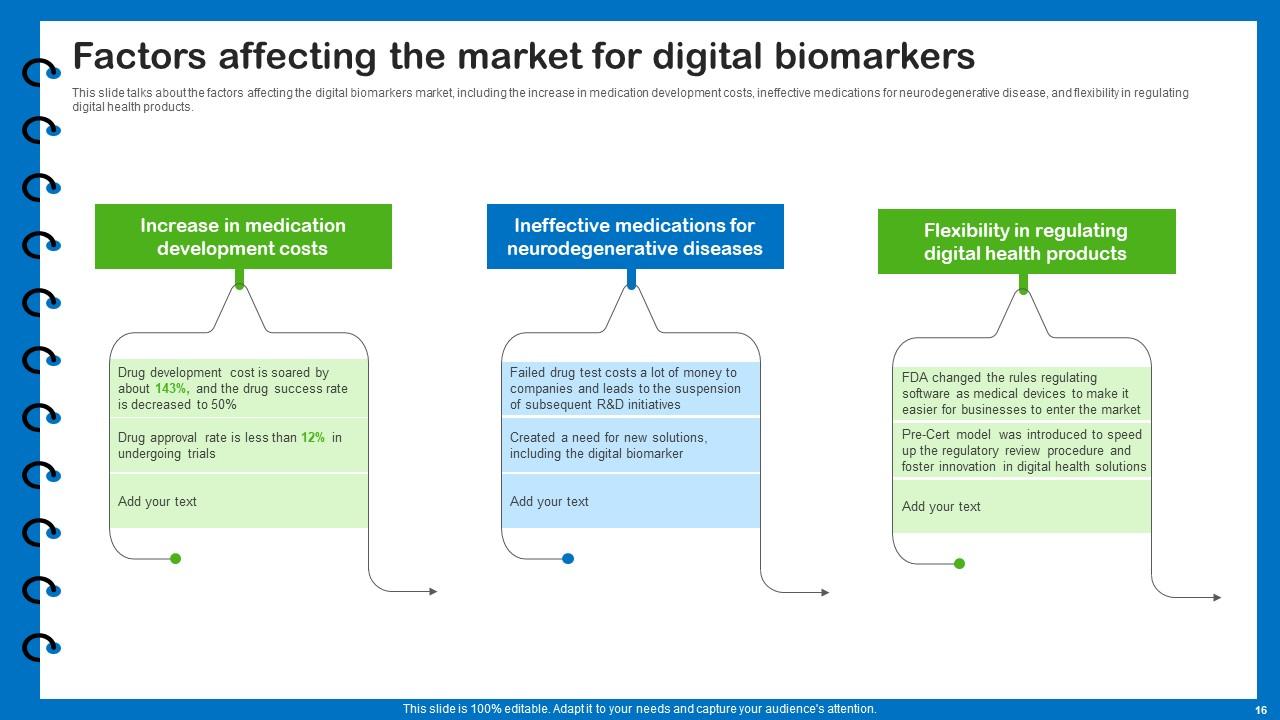
Slide 16: This slide describes factors affecting the digital biomarkers market.
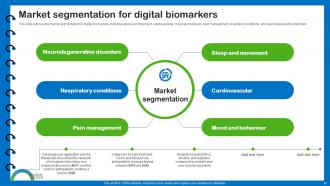
Slide 17: This slide outlines the market segmentation for digital biomarkers.
Slide 18: This slide shows title for topics that are to be covered next in the template.
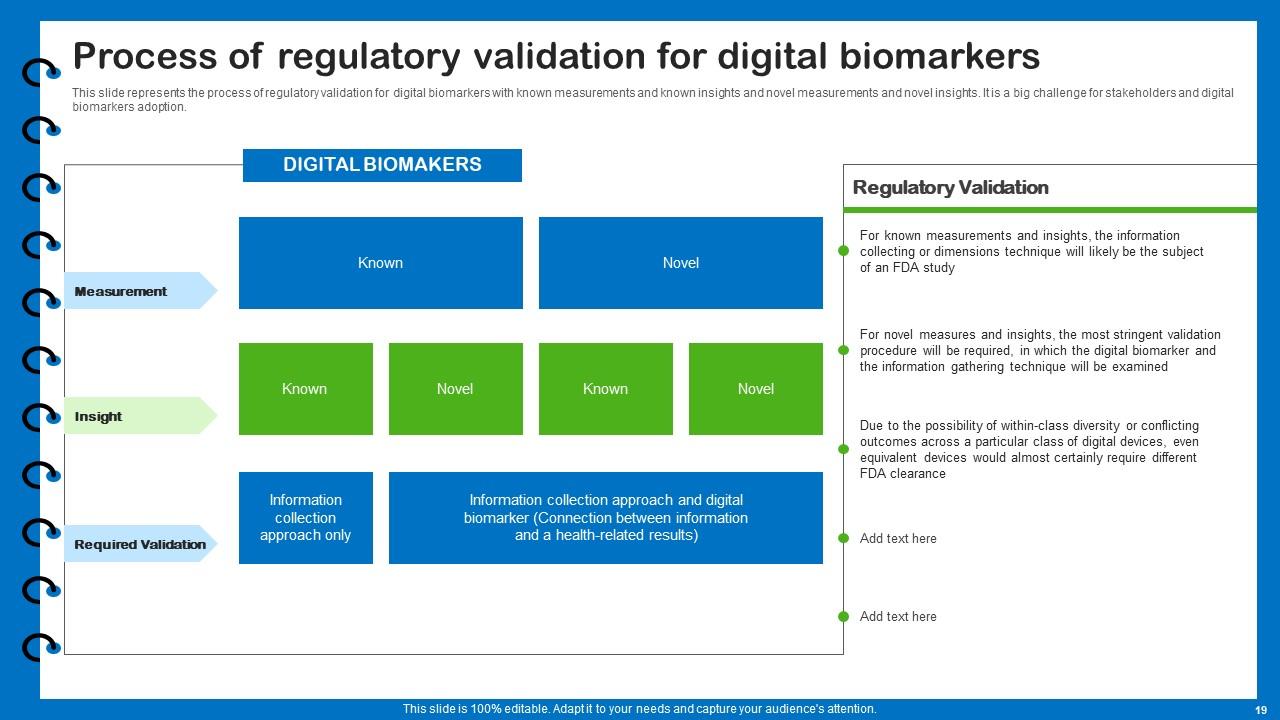
Slide 19: This slide presents the process of regulatory validation for digital biomarkers.
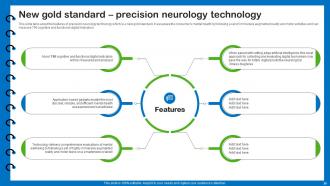
Slide 20: This slide describes the features of precision neurology technology which is a new gold standard.
Slide 21: This slide shows title for topics that are to be covered next in the template.
Slide 22: This slide illustrates the advantages of digital biomarkers in healthcare.
Slide 23: This slide presents how digital biomarkers are transforming the healthcare system.
Slide 24: This slide describes the impact of digital biomarkers on neurology and psychiatry.
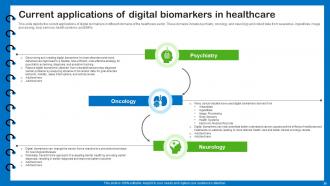
Slide 25: This slide depicts the current applications of digital biomarkers in different domains of the healthcare sector.
Slide 26: This slide shows title for topics that are to be covered next in the template.
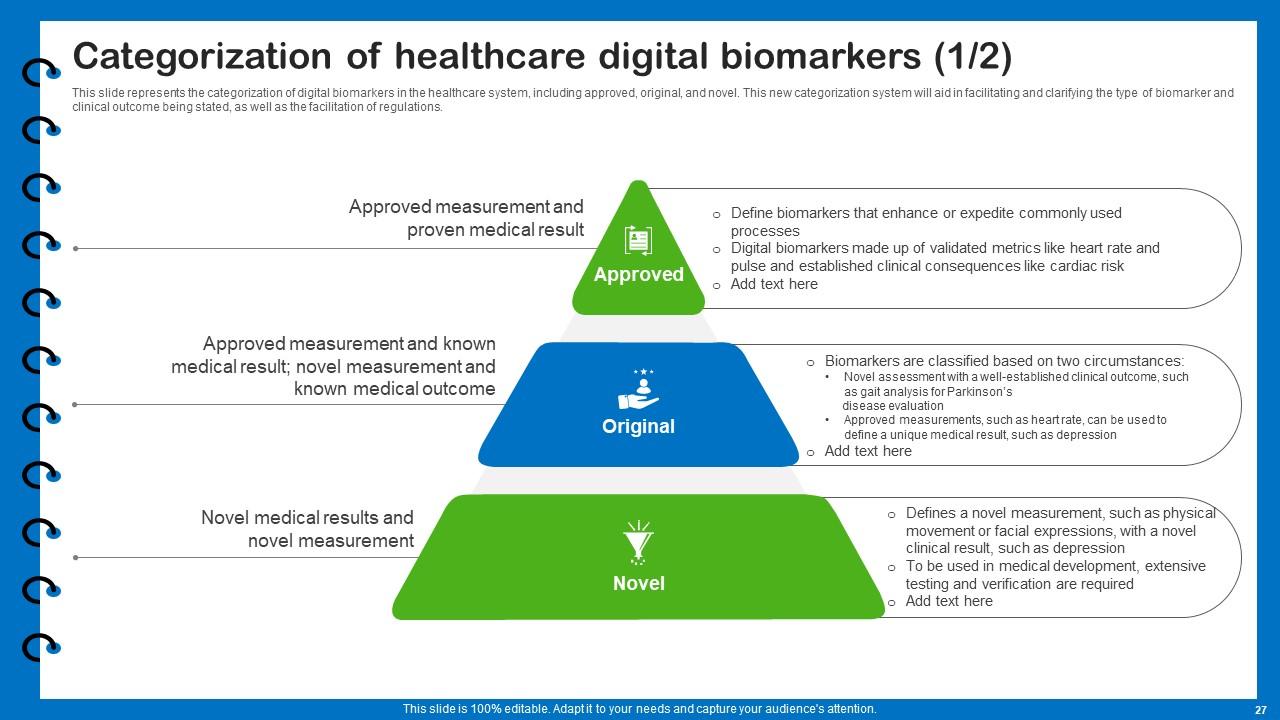
Slide 27: This slide presents the categorization of digital biomarkers in the healthcare system.
Slide 28: This is another slide presenting the categorization of digital biomarkers in the healthcare system.
Slide 29: This slide describes different digital biomarker users.
Slide 30: This slide outlines how digital biomarkers will expand and amplify the user's role.
Slide 31: This slide shows title for topics that are to be covered next in the template.
Slide 32: This slide depicts the enhanced sensing technologies.
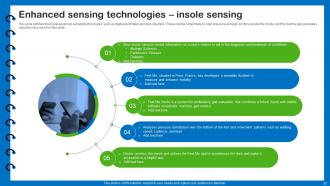
Slide 33: This slide outlines the insole advanced sensing technologies.
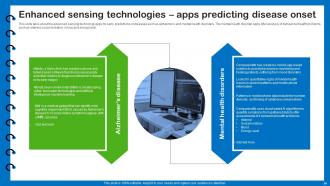
Slide 34: This slide describes the advanced sensing technology apps for early predictions of diseases.
Slide 35: This slide depicts the portable devices used for digital biomarkers and some major players in the industry.
Slide 36: This slide presents use of data analytics to detect and track diseases through sensors.
Slide 37: This slide describes the role of smartphones and artificial intelligence-driven information in digital biomarkers.
Slide 38: This slide shows title for topics that are to be covered next in the template.
Slide 39: This slide illustrates the introduction to the digital biomarker discovery pipeline, an open-source software.
Slide 40: This slide presents the digital biomarker discovery pipeline's landscape.
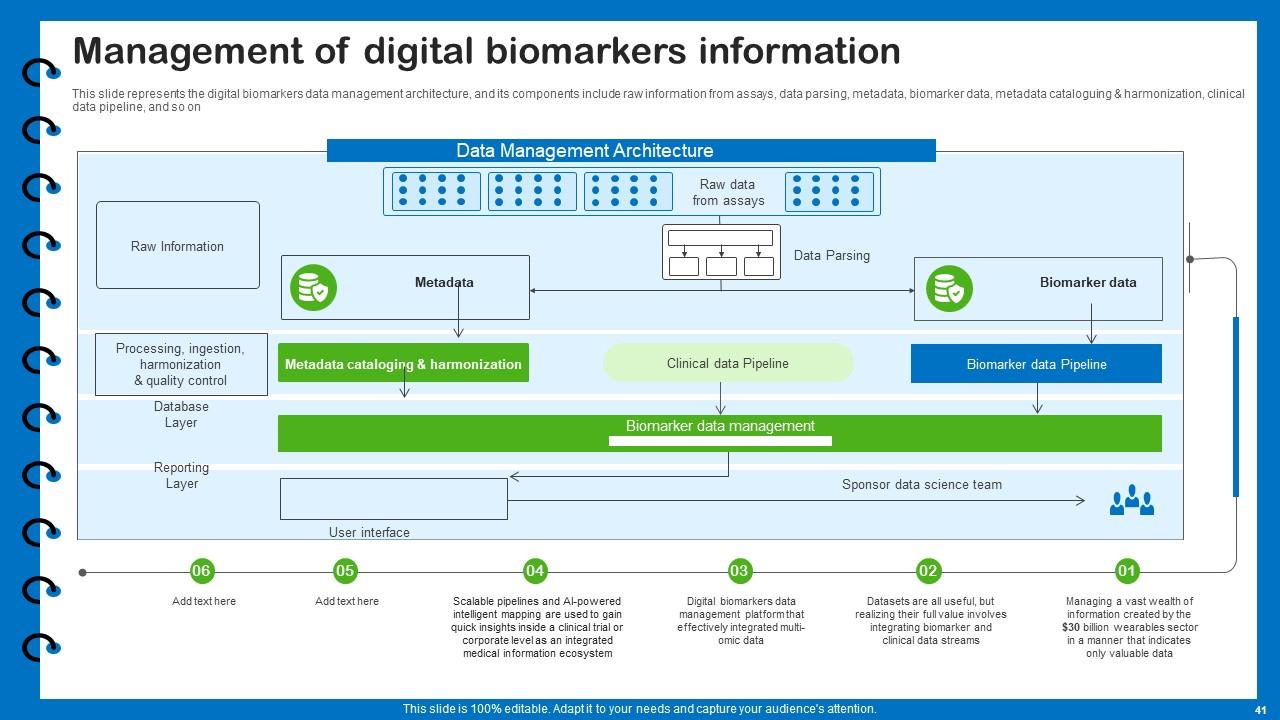
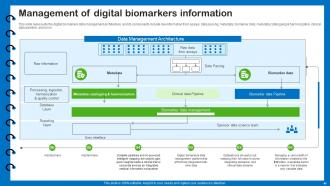
Slide 41: This slide represents the digital biomarkers data management architecture, and its components.
Slide 42: This slide shows title for topics that are to be covered next in the template.
Slide 43: This slide describes the potential use cases of digital biomarkers in biopharma, healthcare providers, and medical insurance payers.
Slide 44: This slide shows title for topics that are to be covered next in the template.
Slide 45: This slide depicts the challenges to digital biomarker adoption.
Slide 46: This slide presents the hurdles before data becomes an insightful digital biomarker.
Slide 47: This slide describes the clinical adoption of digital biomarkers obstacles associated with stakeholder incentives and clinical workflow integration.
Slide 48: This slide talks about the infrastructure hurdles in adopting digital biomarkers.
Slide 49: This slide presents the digital biomarkers adoption challenges related to gold standard validation.
Slide 50: This slide shows title for topics that are to be covered next in the template.
Slide 51: This slide describes the comparison between traditional and digital biomarkers characteristics.
Slide 52: This slide shows title for topics that are to be covered next in the template.
Slide 53: This slide depicts the roadmap for digital biomarkers development.
Slide 54: This slide shows title for topics that are to be covered next in the template.
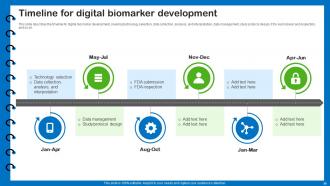
Slide 55: This slide describes the timeline for digital biomarker development.
Slide 56: This slide shows title for topics that are to be covered next in the template.
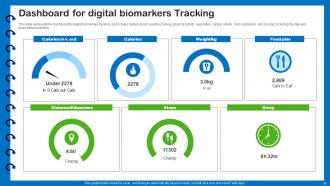
Slide 57: This slide presents the dashboard for digital biomarkers tracking.
Slide 58: This slide shows all the icons included in the presentation.
Slide 59: This slide is titled as Additional Slides for moving forward.
Slide 60: This slide presents Brief history from telematics to digital health.
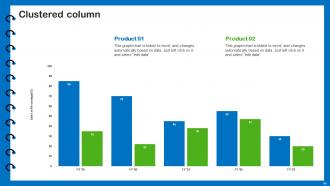
Slide 61: This slide provides Clustered Column chart with two products comparison.
Slide 62: This slide depicts Area chart with two products comparison.
Slide 63: This slide depicts Venn diagram with text boxes.
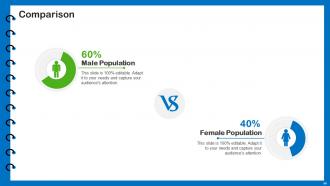
Slide 64: This is a Comparison slide with additional textboxes and related imagery.
Slide 65: This slide shows Post It Notes. Post your important notes here.
Slide 66: This slide presents Roadmap with additional textboxes.
Slide 67: This slide contains Puzzle with related icons and text.
Slide 68: This is Our Goal slide. State your firm's goals here.
Slide 69: This is a Thank You slide with address, contact numbers and email address.
Health Information Management Powerpoint Presentation Slides with all 74 slides:
Use our Health Information Management Powerpoint Presentation Slides to effectively help you save your valuable time. They are readymade to fit into any presentation structure.
FAQs
Digital biomarkers are objective, quantifiable physiological and behavioral measurements collected through digital devices. They are transforming the healthcare system by providing real-time, continuous data that can be used for early disease detection, personalized treatment, and monitoring patient outcomes.
The principles of behavioral analysis using digital biomarkers involve capturing and analyzing data related to an individual's behavior, such as activity levels, sleep patterns, and social interactions. These biomarkers can provide insights into a person's mental and physical well-being, enabling personalized interventions and treatment plans.
Direct digital biomarkers are measured directly from the physiological or behavioral signals of an individual, such as heart rate or sleep quality. Indirect digital biomarkers, on the other hand, are derived from the analysis of multiple direct biomarkers or other contextual data, providing insights into specific health conditions or behaviors.
The regulatory validation process for digital biomarkers involves demonstrating their safety, efficacy, and clinical utility. It typically includes rigorous testing, clinical trials, and obtaining regulatory approvals or clearances from relevant authorities, such as the FDA in the United States.
Several factors can influence the digital biomarkers market, including advancements in technology, regulatory policies, healthcare infrastructure, adoption rates, reimbursement policies, and collaborations between stakeholders. These factors can shape the market dynamics and growth potential of digital biomarkers.
-
Every time I ask for something out-of-the-box from them and they never fail in delivering that. No words for their excellence!
-
No second thoughts when I’m looking for excellent templates. SlideTeam is definitely my go-to website for well-designed slides.