Drug discovery and development processes to access potential product toxicity complete deck
Our Drug Discovery And Development Processes To Access Potential Product Toxicity Complete Deck are topically designed to provide an attractive backdrop to any subject. Use them to look like a presentation pro.
Our Drug Discovery And Development Processes To Access Potential Product Toxicity Complete Deck are topically designed to p..
- Google Slides is a new FREE Presentation software from Google.
- All our content is 100% compatible with Google Slides.
- Just download our designs, and upload them to Google Slides and they will work automatically.
- Amaze your audience with SlideTeam and Google Slides.
-
Want Changes to This PPT Slide? Check out our Presentation Design Services
- WideScreen Aspect ratio is becoming a very popular format. When you download this product, the downloaded ZIP will contain this product in both standard and widescreen format.
-

- Some older products that we have may only be in standard format, but they can easily be converted to widescreen.
- To do this, please open the SlideTeam product in Powerpoint, and go to
- Design ( On the top bar) -> Page Setup -> and select "On-screen Show (16:9)” in the drop down for "Slides Sized for".
- The slide or theme will change to widescreen, and all graphics will adjust automatically. You can similarly convert our content to any other desired screen aspect ratio.
Compatible With Google Slides

Get This In WideScreen
You must be logged in to download this presentation.
PowerPoint presentation slides
Deliver an informational PPT on various topics by using this Drug Discovery And Development Processes To Access Potential Product Toxicity Complete Deck. This deck focuses and implements best industry practices, thus providing a birds eye view of the topic. Encompassed with fourty slides, designed using high quality visuals and graphics, this deck is a complete package to use and download. All the slides offered in this deck are subjective to innumerable alterations, thus making you a pro at delivering and educating. You can modify the color of the graphics, background, or anything else as per your needs and requirements. It suits every business vertical because of its adaptable layout.
People who downloaded this PowerPoint presentation also viewed the following :
Content of this Powerpoint Presentation
Slide 1: This title slide introduces the Drug Discovery and Development Processes to Access Potential Product Toxicity. Add the name of your company here.
Slide 2: This is the Agenda slide for Drug Discovery and Development Processes to access Potential Product Toxicity.
Slide 3: It contains the Table of Contents. It includes - Overview of Drug Discovery and Development Process, Drug Discovery and Development Process in Detail, Drug Discovery and Development – Reasons for Drug Failure, etc.
Slide 4: This is a table of content slide showing the Drug Discovery and Development Objectives, Elements in creating New Drugs, Responsibilities of Drug Discovery and Development Committee, Responsibilities of Product Development Team, and Drug Discovery and Development Approaches.
Slide 5: This slide presents the Objectives of Drug Discovery and Development. It shows the major objectives of drug discovery and development in the organization such as promotion and development of the new drugs, identifying clinical and pre-clinical investigators in drug development, determining a starting safe dose for first-in-human study, etc.
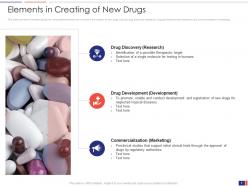
Slide 6: This slide presents the Elements in Creating New Drugs. It provides information about the various elements that are involved in the creation of new drugs such as drug discovery (research), drug development (development), and commercialization (marketing).
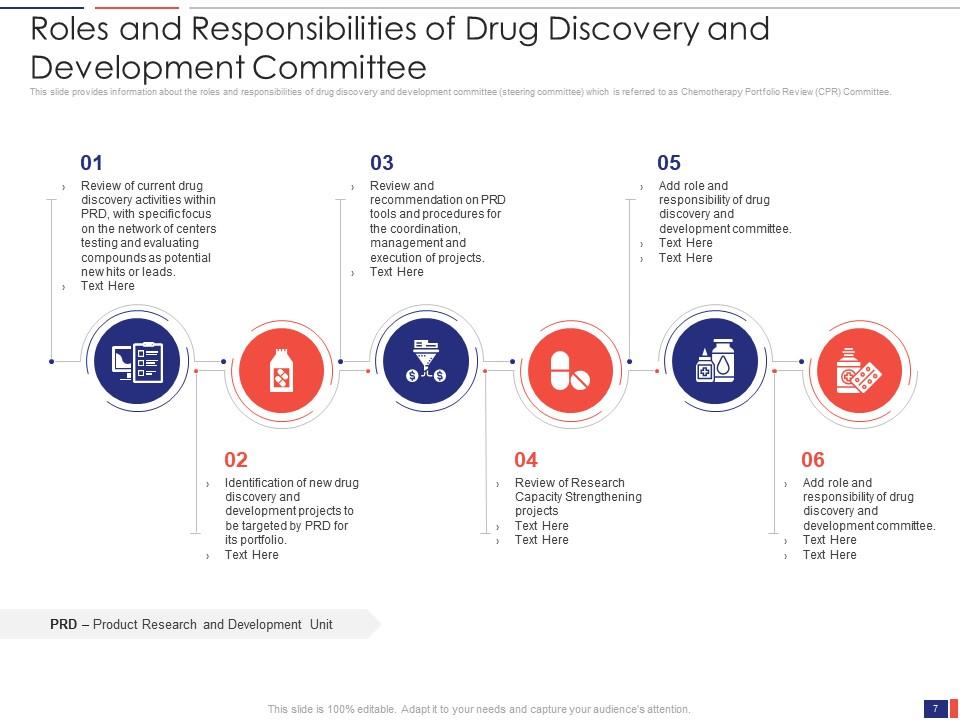
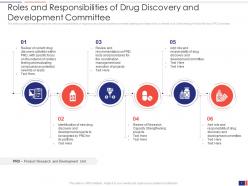
Slide 7: This slide presents the Roles and Responsibilities of the Drug Discovery and Development Committee. It provides information about the roles and responsibilities of the drug discovery and development committee (steering committee) which is referred to as the Chemotherapy Portfolio Review (CPR) Committee.
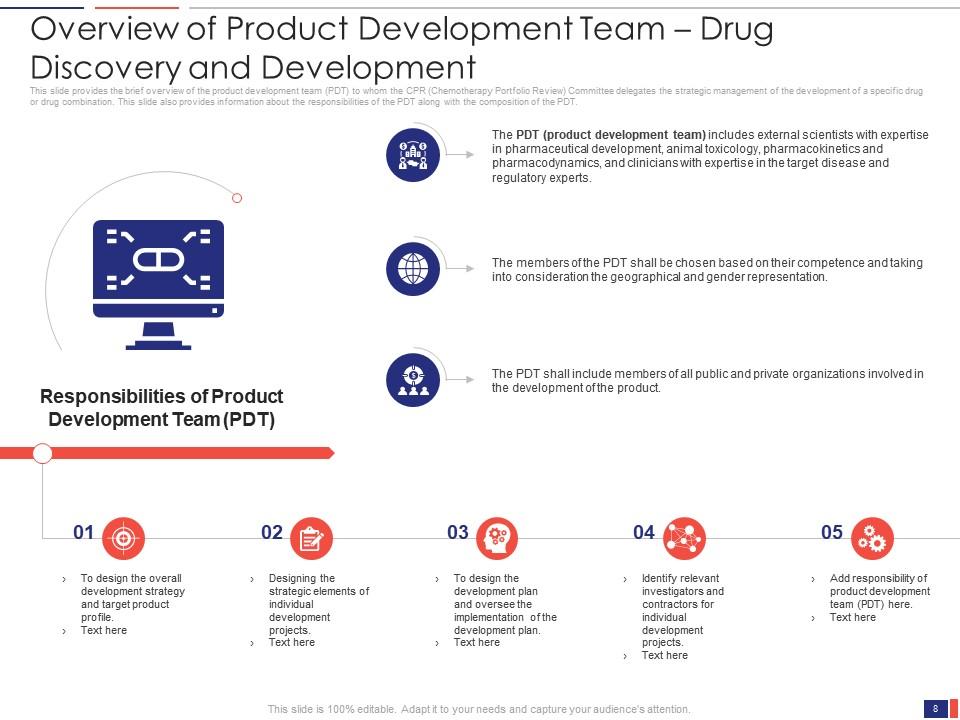
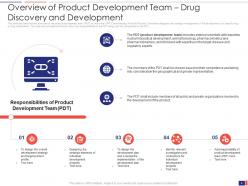
Slide 8: This slide presents the Overview of Product Development Team – Drug Discovery and Development. It provides a brief overview of the product development team (PDT) to whom the CPR (Chemotherapy Portfolio Review) Committee delegates the strategic management of the development of a specific drug or drug combination.
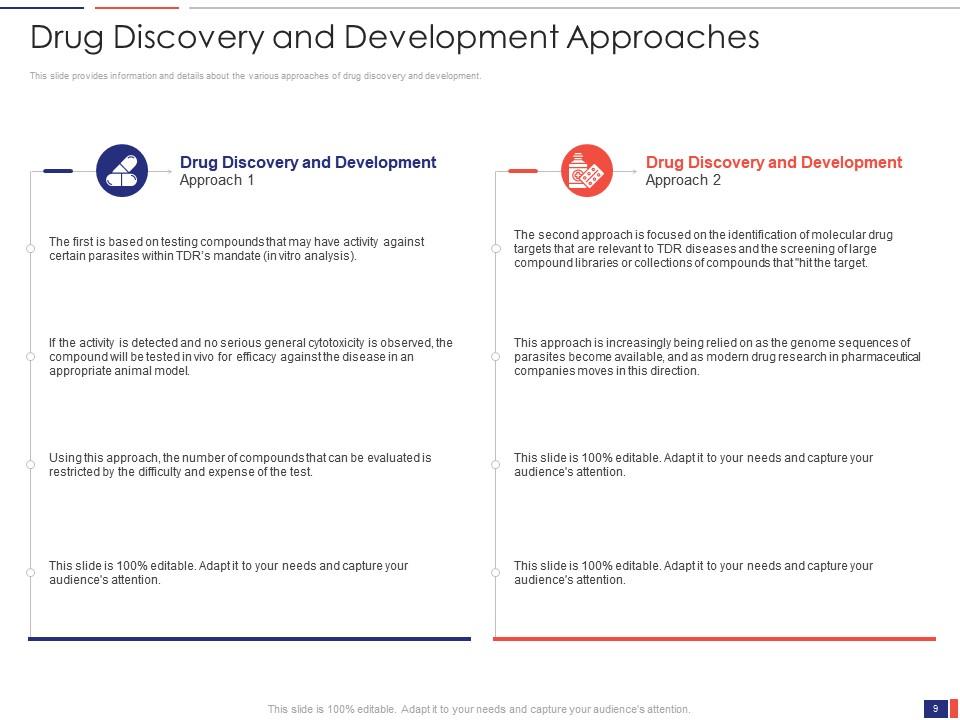
Slide 9: This slide presents the Drug Discovery and Development Approaches. It provides information and details about the various approaches to drug discovery and development.
Slide 10: This is a table of content slide showing the Overview of the Drug Discovery and Development Process.
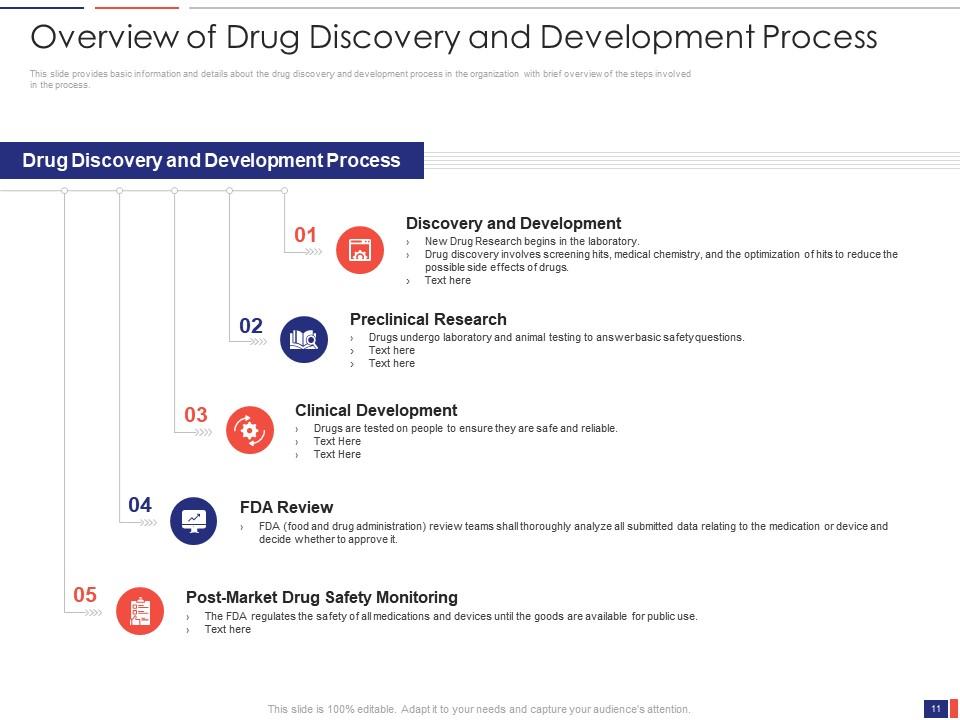
Slide 11: This slide presents the Overview of the Drug Discovery and Development Process. It provides basic information and details about the drug discovery and development process in the organization with a brief overview of the steps involved in the process.
Slide 12: This is a table of content slide showing the Drug Discovery and Development Process in Detail.
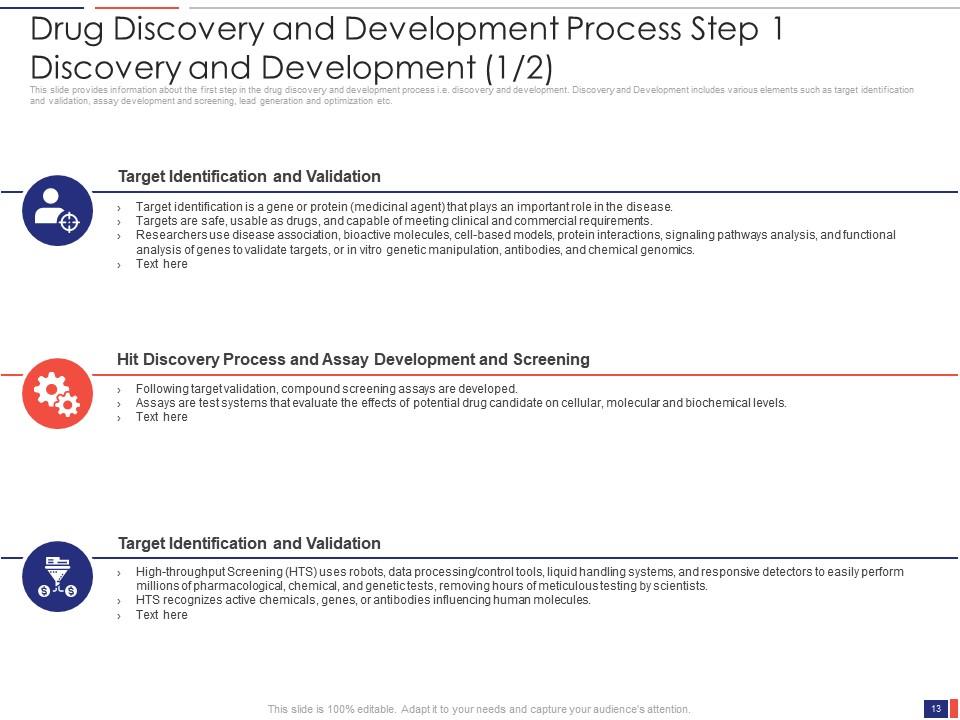
Slide 13: This slide presents the Drug Discovery and Development Process Step 1- Discovery and Development (1/2). It provides information about the first step in the drug discovery and development process i.e. discovery and development.
Slide 14: This slide presents the Drug Discovery and Development Process Step 1- Discovery and Development (2/2). Discovery and Development include various elements such as target identification and validation, assay development and screening, lead generation and optimization, etc.
Slide 15: This slide presents the Drug Discovery and Development Process Step 2 – Preclinical Research (1/2). It provides information about the second step in the drug discovery and development process i.e. preclinical research.
Slide 16: This slide presents the Drug Discovery and Development Process Step 2 – Preclinical Research (2/2). Preclinical Research includes various elements such as absorption, distribution, disposition, metabolism, in vivo, in vitro, ex vivo assays, in silico assays, drug delivery methods, etc.
Slide 17: This slide presents the Drug Discovery and Development Process Step 3 – Clinical Development (1/3). It provides information about the third step in the drug discovery and development process i.e. clinical development.
Slide 18: This slide presents the Drug Discovery and Development Process Step 3 – Clinical Development (2/3). Clinical Development includes various elements such as dose escalation, single ascending, healthy volunteer study, biological samples collection, pharmacokinetic analysis, feces sample analysis for drug, patient protection, etc.
Slide 19: This slide presents the Drug Discovery and Development Process Step 3 – Clinical Development (3/3). Bioanalytical methods detect analytes and metabolites such as drugs or biomarkers in biological or human samples to assess the effectiveness and safety of drugs.
Slide 20: This slide presents the Drug Discovery and Development Process Step 4 – FDA Review (1/2). It provides information about the fourth step in the drug discovery and development process i.e. FDA (food and drug administration) review.
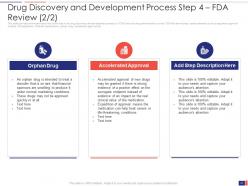
Slide 21: This slide presents the Drug Discovery and Development Process Step 4 – FDA Review (2/2). FDA Review includes various elements such as regulatory approval timeline, IND application, NDA/BLA applications, orphan drug, accelerated approval, etc.
Slide 22: This slide presents the Drug Discovery and Development Process Step 5 – Post-Market Drug Safety Monitoring (1/2). It provides information about the fifth step in the drug discovery and development process i.e. Post-Market Monitoring.
Slide 23: This slide presents the Drug Discovery and Development Process Step 5 – Post-Market Drug Safety Monitoring (2/2). Post-market Monitoring includes various elements such as supplemental applications, manufacturer inspections, drug advertising, generic drugs, active surveillance, etc.
Slide 24: This is a table of content slide showing the Drug Discovery and Development – Reasons for Drug Failure.
Slide 25: This slide presents the Drug Discovery and Development – Reasons for Drug Failure. It provides information about the various reasons for drug failure in drug discovery and development such as high toxicity, efficacy, PK properties or poor bioavailability, inadequate drug performance, etc.
Slide 26: This is a table of content slide showing the Drug Discovery and Development Concepts.
Slide 27: This slide presents the Drug Discovery and Development Concepts. It provides information about some relevant drug discovery and development concepts such as drug master files, drugs for pediatric use, drugs for veterinary use, small molecule, biologics, etc.
Slide 28: This is a table of content slide showing the Drug Discovery and Development Activity Chart.
Slide 29: This slide presents the Drug Discovery and Development Process Funnel. It provides information about the drug discovery and development funnel, with details regarding drug discovery, clinical trial phases, FDA review, approved drug details, post-market drug safety monitoring details, etc.
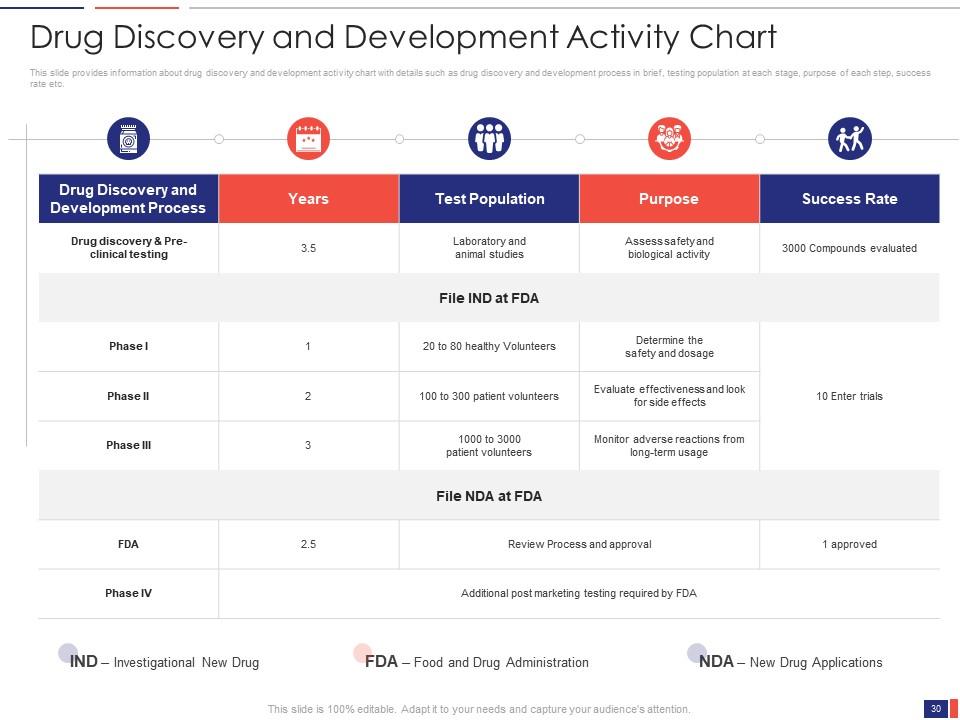
Slide 30: This slide presents the Drug Discovery and Development Activity Chart. It provides information about drug discovery and development activity chart with details such as drug discovery and development process, in brief, testing population at each stage, the purpose of each step, success rate, etc.
Slide 31: This is the Icons Slide for Drug Discovery and Development Processes to access Potential Product Toxicity.
Slide 32: This slide presents the Additional Slides.
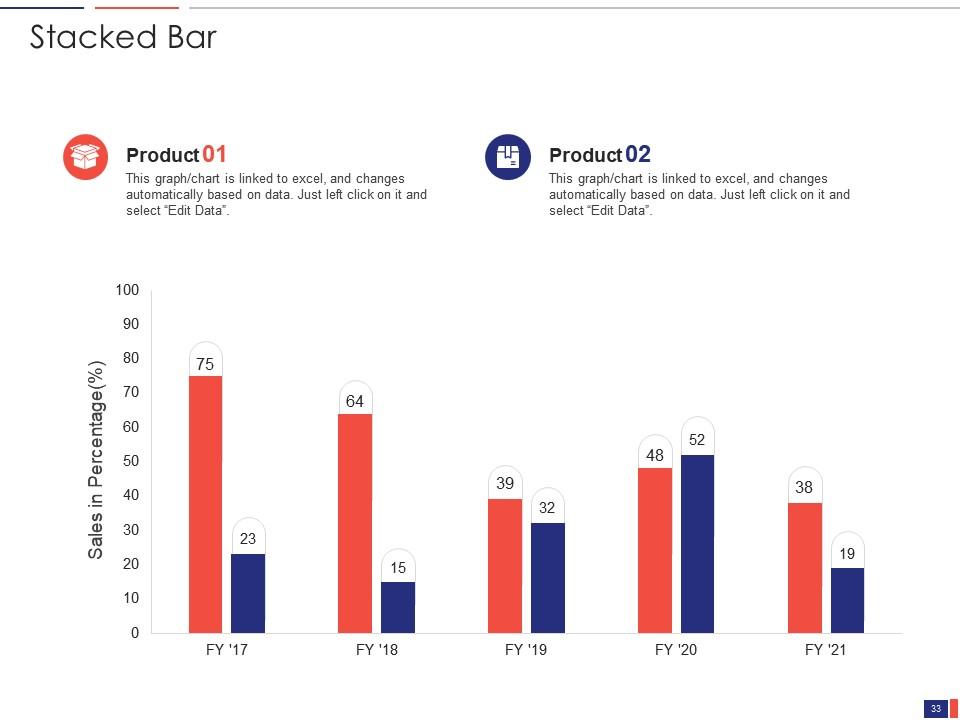
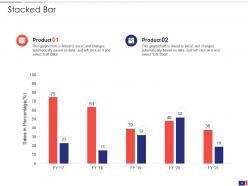
Slide 33: This slide shows a Stacked Bar that compares 2 products’ sales over a timeline of financial years.
Slide 34: This slide contains Post It Notes that can be used to express any brief thoughts or ideas.
Slide 35: This slide presents the Circular Process.
Slide 36: This slide presents the Dashboard with the data’s numbers at Low, Medium, and High.
Slide 37: This slide is a Timeline template to showcase the progress of the steps of a project with time.
Slide 38: This is the Puzzle slide.
Slide 39: This slide is a Roadmap template to showcase the stages of a project, for example.
Slide 40: This is a Thank You slide where details such as the address, contact number, email address are added.
Drug discovery and development processes to access potential product toxicity complete deck with all 40 slides:
Use our Drug Discovery And Development Processes To Access Potential Product Toxicity Complete Deck to effectively help you save your valuable time. They are readymade to fit into any presentation structure.
-
Design layout is very impressive.
-
Excellent template with unique design.
-
Out of the box and creative design.
-
Commendable slides with attractive designs. Extremely pleased with the fact that they are easy to modify. Great work!